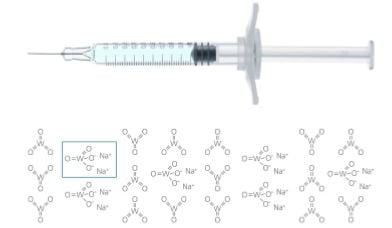
Drugs based on technologically manufactured active ingredients filled into syringes currently have a share of approximately 15% of the total market value for pre-filled syringes. These high-growth biotech drugs are sensitive with regard to possible interactions with individual syringe components. Syringe system manufacturers therefore strive for a reduction or avoidance of syringe components like silicone oil or tungsten pin. This article by Bernd Zeiss, Manager Technical Support Medical Systems at Gerresheimer Bu?nde GmbH, tackles the issue of reduction and avoidance of tungsten in prefillable glass syringes.