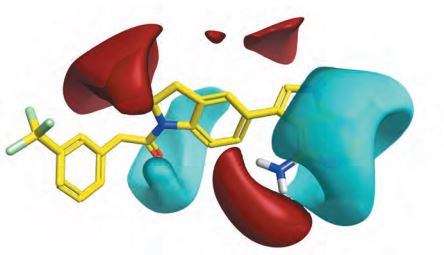
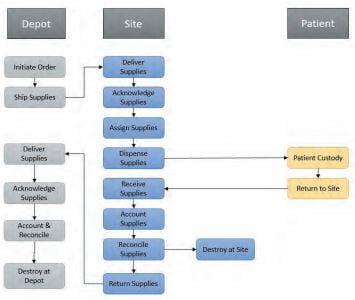
The BioRegion of Catalonia: What’s Behind the European Country with More Pharma Companies per Capita?
Last autumn, Barcelona hosted the CPhI Worldwide for the
first time, and it was a record-breaking year for the event.
In their article, Nuria Pelaez, Anna Rovira and...